ProBio
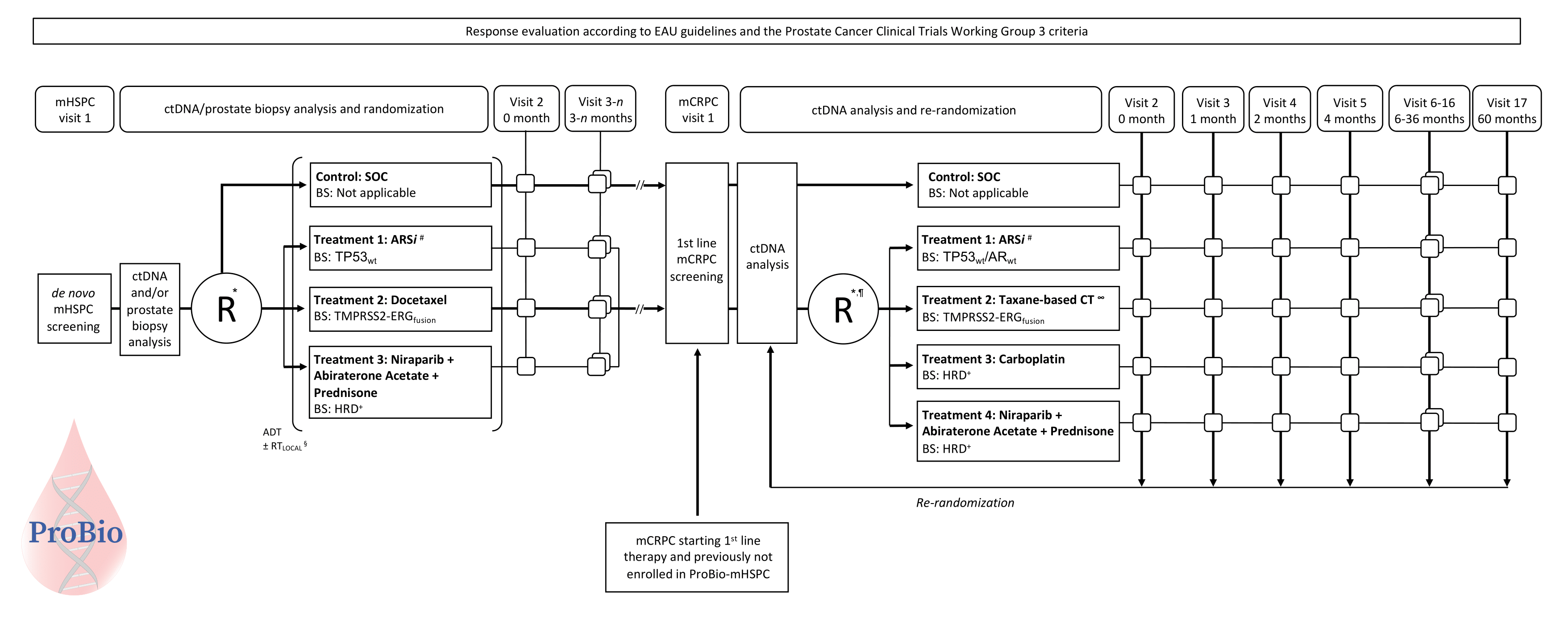
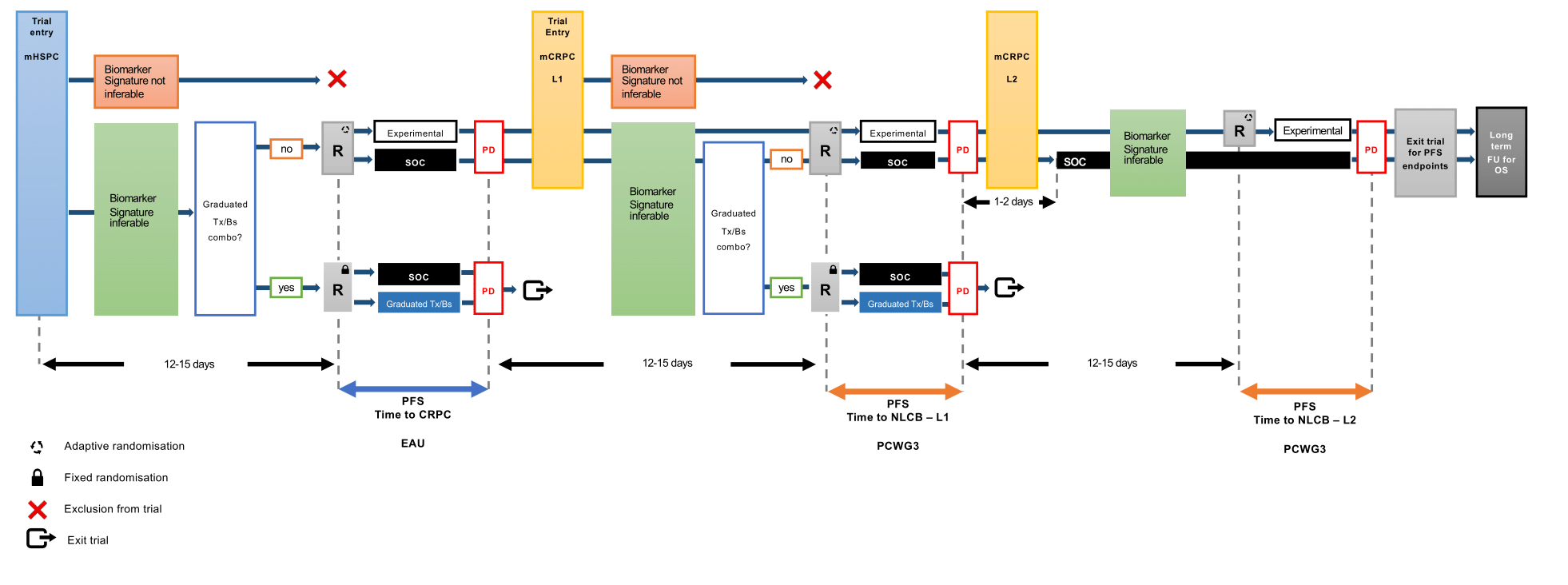
ProBio is an outcome-adaptive, multi-arm, open-label, multiple assignment randomized biomarker driven platform trial in patients with metastatic prostate cancer. Patients will be randomized to control or experimental treatment arms. Patients in the control arm will receive standard of care following national guidelines. Patients in the experimental arm will be randomized to treatments based on a biomarker signature inferred from diagnostic tissue or liquid biopsy profiling. The predefined biomarker signatures are tumor properties or mutations in genes/pathways with previously demonstrated clinical validity (e.g. prognostic value or association with treatment response). The biomarker signatures are identified using a hybridisation capture gene panel specifically designed for prostate cancer.

The colours of the patients with metastatic prostate cancer represent different biomarker profiles. After written informed consent, archival diagnostic tissue tumor blocks are collected and/or peripheral blood is drawn. Extractions is performed to obtain DNA from diagnostic biopsy specimens and/or cell-free DNA from plasma, and germline DNA from white blood cells. A fraction of the DNA from diagnostic biopsy and cell-free DNA from plasma originates from cancer cells, so called tumor- or circulating tumor DNA, respectively. Targeted sequencing is conducted to analyse somatic alterations and relevant germline variants. This information is condensed into a biomarker signature report which in turn is used for randomisation of the study participants. The randomisation takes the biomarker signature into account in the treatment arms. In the control arm standard of care is given according to national guidelines without knowledge about the biomarker signatures. Progression (disease relapse) is evaluated according to established international standards (i.e. European Association for Urologu (EAU) and Prostate Cancer Working Group version 3 guidelines). Study participants who progress are reanalysed and rerandomised. Outcomes data is continuously used to update the randomisation probabilities to identify treatment–biomarker signature combinations that "graduate" (i.e. superior compared to standard of care) or are terminated for futility.